
The critically endangered Amphibians from the small Escudo de Veraguas Island Panama
Olmedo Santiago1 Astrid Lizondro2-3 Marcos Ponce4
https://orcid.org/0009-0001-3065-2398 https://orcid.org/0000-0002-8781-6613 https://orcid.org/0000-0002-6850-178X
Víctor Acosta-Chaves5 Abel Batista2-3
https://orcid.org/0000-0002-6126-622X https://orcid.org/0000-0001-8053-3373
1Universidad de Panamá, Centro Regional Universitario de Bocas del Toro, Panamá
2Universidad Autónoma de Chiriquí, 0427 David, Chiriquí, Panamá
3Fundación Los Naturalistas, P.O. Box 0426-01459. David, Chiriquí, Panamá
4Bioconsultant, David, Chiriquí, Panamá
5Universidad de Costa Rica. Sede del Atlántico. Recinto Paraíso, Cartago, Costa Rica.
Corresponding author: abel.batista@unachi.ac.pa
Enviado el 5 de octubre de 2023. Aceptado el 29 de Noviembre de 2023.
https://doi.org/10.59722/rcvn.v1i2.708
Abstract
Escudo de Veraguas, a small island in Western Panama, is home to the maritime worm salamander Oedipina maritima and the Costa Rica Stream-welling Frog Craugastor ranoides, both species are in continuing decline in the extent and quality of their habitat. During three monitoring surveys of the island, 50 plots were installed, 25 in forest and 25 in swamps. The population was estimated at 88 ind./ha for O. maritima and 59 ind./ha for C. ranoides. As a conservation measure, we recommend long-term monitoring to guarantee the survival of these Critically Endangered species.
Keywords
Climate change, Craugastor ranoides, habitat degradation, Oedipina maritima
Resumen
Escudo de Veraguas es una pequeña isla en el oeste de Panamá, hogar de la
salamandra lombriz marítima Oedipina maritima y la rana de los arroyos Craugastor
ranoides, ambas especies están en continuo declive en la extensión y
calidad de su hábitat. Durante tres estudios de monitoreo en la isla, se
instalaron 50 parcelas, 25 en hábitat forestal y 25 en pantanos. La población
se estimó en 88 ind./ha para O. maritima y 59 ind./ha para C.
ranoides. Como medida de conservación, recomendamos realizar estudios de seguimiento
a largo plazo para garantizar la supervivencia de estas especies en Peligro
Crítico.
Palabras clave
Cambio climático, Craugastor ranoides, degradación del hábitat, Oedipina maritima
Introduction
Despite its small size (4 km2) in Escudo de Veraguas, there are 13 species of amphibians (Batista, 2015, unpublished data). The isolation of this land mass, about 3.5-1.8 million years ago (Coates et al. 2005) has led to the speciation of some species that until now are known to be endemic to the island. This is the case of the maritime worm salamander (Oedipina maritima), a critically endangered (CR) species of which there is only one record and very little is known about its biology (García-París & Wake, 2000; Zumbado-Ulate et al. 2011; IUCN SSC Amphibian Specialist Group 2020b). The island is also home to the CR Costa Rica Stream-dwelling Frog (Craugastor ranoides), a species for which about 80 % of its populations have disappeared (IUCN SSC Amphibian Specialist Group, 2020), with only one stable population in the Guanacaste Conservation Area of Costa Rica (Zumbado-Ulate et al. 2011). Currently, these species are under continuing decline in the extent and quality of their habitat (IUCN SSC Amphibian Specialist Group 2020a, b). This work aimed to estimate the population status and conservation of the O. maritima and C. ranoides in Isla Escudo de Veraguas, Panama.
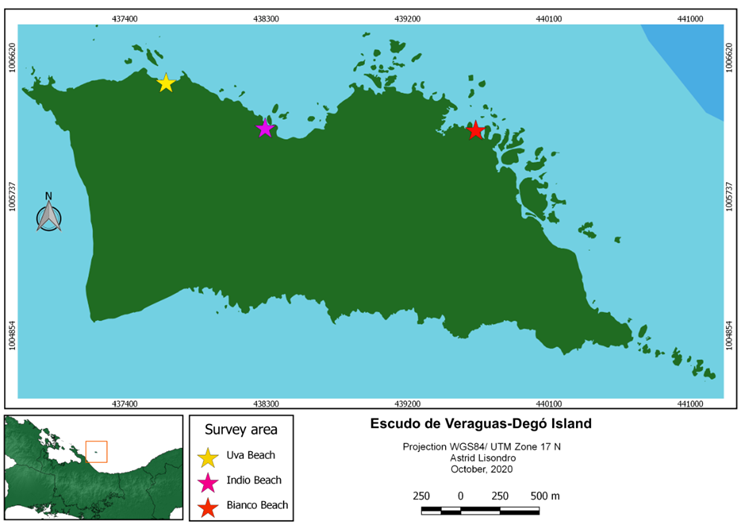
Figure 1.
Map of Escudo de Veraguas Island, showing the three surveyed areas.
Materials and Methods
Study site
Three surveys were conducted to Escudo de Veraguas Island (figure 1) Comarca Ngäbe Buglé, Panama (9.0984° N, 81.5564° W), on September, October 2019, and March 2020, visiting three different sites on each trip, to the beaches Uva, Indio and Bianco, at the North and Northeast part of the island, all with the same type of ecosystem, the lowland tropical forest (hereafter: forest; figure 2A) and the Freshwater swamp forest (hereafter: swamp, figure 2B). The sampling was carried out in two types of habitats: forest and swamp. The forest habitat is mainly composed of hilly areas, and large trees up to more than 20 m in height, from 100 to 200 m from the coastal zone. The swamp area consisted of flooded areas in contact with the coastal zone, mainly with the presence of ferns, mangroves and palm trees (Cocos nucifera and Manicaria saccifera).
Density and habitat occupancy
To evaluate the density of the species we set 50 plots of 8x8 m, located in each habitat, 25 in the swamp and 25 in the forest, each plot was revised once, by two to three persons walking in zigzag, turning logs, checking epiphytes and sites suitable to the presence of these animals (Heyer et al. 2001). The number of individuals and ecological data of the species was recorded. To estimate the relative density, the total of individuals found was divided by the total area among plots. We use the program PRESENCE 2.13.12 to estimate patch occupancy and the probability of detecting at least one individual in every plot on the three different surveys, using a single season occupancy model (Hines, 2006; MacKenzie et al. 2002).
Results and Discussion
Estimations of the populations showed a relative density of 88 ind./ha for O. maritima (figure 2C) and 59 ind./ha for C. ranoides (figure 2D). There were no differences in the number of individuals found in both habitats (Chi2=1.04; p>0.05; table 1). The two types of habitat models, forest (psi(forest.), p(.); 30%) and swamp (psi(swamp),p(.), 35%) explain the 65% of the variation found in the habitat occupancy analysis for O. maritima (table 2), while the individual detection model (psi(.),p(.); 42 %) with the swamp habitat model (psi(swamp.),p(.); 32%) explained the 74 % of the variation for the analysis for C. ranoides
(table 3). The probabilities of occupancy for O. maritima, was 50% (SD: 0.0) in forest and 25% (SD: 0.17) in swamp. For C. ranoides the probabilities of occupancy, was 100% (SD: 0.0) in both types of habitats.
Table 1.
Detailed information of amphibians found on each type of habitat at Escudo de Veraguas Island, Comarca Ngäbe Blugé, Panama
|
Habitat |
Trait |
Oedipina maritima |
Craugastor ranoides |
|
Forest |
Total indiv. |
13 |
6 |
|
|
plots with records |
7 |
5 |
|
Swamp |
Total indiv. |
15 |
13 |
|
plots with records |
3 |
6 |
|
|
Overall |
Total indiv. |
28 |
19 |
|
|
plots with records |
10 |
11 |
Before this study, the Oedipina maritima was known from only eight adult specimens and two clutches of eggs, used for the description of the species (García-París & Wake 2000). We have been able to observe 28 individuals and seems that it could prefer a certain type of microhabitat. In a single plot, we found 13 individuals in the swamp habitat, close to the coastal line, in coconut palm grove and decomposing palm leaves. We also have found a concentration of specimens around coconut palm grove, in a more recent expedition (in August 2021) we found five individuals during a 10 minutes search in about 1 m2.

Figure 2.
Surveyed habitat and the critically endangered species from Escudo de Veraguas Island. A) Forest; B) swamp; C) Maritime Worm Salamander (Oedipina marítima); D) Costa Rica Stream dwelling Frog (Craugastor ranoides).
Table 2.
Occupancy modeling for Maritime Worm Salamander (Oedipina marítima) in Escudo de Veraguas Island
|
Model |
AIC |
deltaAIC |
AIC wgtModel |
Likelihood |
no.Par. |
|
psi(.),p(.) |
56.69 |
0.00 |
0.4212 |
1.0000 |
2 |
|
psi(swamp.),p(.) |
57.23 |
0.54 |
0.3215 |
0.7634 |
2 |
|
psi(forest+swamp.),p(.) |
58.40 |
1.71 |
0.1791 |
0.4253 |
3 |
|
psi(forest.),p(.) |
60.06 |
3.37 |
0.0781 |
0.1854 |
2 |
Table 3.
Occupancy modeling for Costa Rica stream-dwelling frog (Craugastor ranoides) in Escudo de Veraguas Island
|
Model |
AIC |
deltaAIC |
AIC wgtModel |
Likelihood |
no.Par. |
|
psi(swamp),p(.) |
50.12 |
0.00 |
0.3474 |
1.0000 |
2 |
|
psi(forest.),p(.) |
50.37 |
0.25 |
0.3066 |
0.8825 |
2 |
|
psi(forest+swamp),p(.) |
51.46 |
1.34 |
0.1778 |
0.5117 |
3 |
|
psi(.),p(.) |
51.57 |
1.45 |
0.1683 |
0.4843 |
2 |
Although Craugastor ranoides have been usually found along fast-flowing streams and usually sit on boulders (Zumbado-Ulate et al. 2011; Puschendorf et al. 2019), in Escudo de Veraguas Island, there are
no fast-flowing streams, neither rocks, instead slow flowing streams or merely stagnant water areas. The frog was found also far from any water source in the forest plots. Although C. ranoides is a stream dwelling species, it has been reported also far from streams during rainy season (Zumbado-Ulate et al. 2011). However, the climate around the Escudo de Veraguas island is more humid than the North Pacific
side of Costa Rica, without a marked season throughout the year. The habitat occupation was similar for both types of habitats during all the survey.
Very few populations of C. ranoides appears to be stable (Puschendorf et al. 2019) and it has declined in mostly all areas of its distribution, with last known records for populations at Península de Santa Elena, Costa Rica and Escudo de Veraguas Island in Panama (this study). Seems that these two populations could be refuges for the species. However, there is a need to corroborate its identity through molecular genetic analysis. Given that a genetic variation and haplotype differentiation, have been found within populations in Santa Elena region. There is evidence of a close phylogenetic relationship with the related species C. evanesco of the C. punctariolus species group from Panama, with samples of the Cerro Cacao (cloud forest), near to the Santa Elena dry forest (Puschendorf et al. 2019). Then, the inclusion of any other population of C. ranoides from the rest of its distribution in molecular analysis is determinant to establish effective conservation actions.
State of conservation
Unfortunately, one of the habitats with presence of both species is located, the swamp, is right in the transition between the flooded area and the beach, which is also the site of greatest public use on the island. This area is used also by local fishermen who stay temporarily on the island (usually between August to October), they cut the leaves of the palms and trees for the construction of ranches, the excessive cutting of these leaves and trees every year, could significantly increase the entry of sunlight and have consequences in the biology and distribution of these amphibians.
There are urgent measures that must be taken to guarantee the conservation of the species that inhabit the island. In the first place, make an exhaustive and detailed evaluation of the diversity present in the area, also it is a priority to carry out molecular genetic studies to determine the relationship between the island species with those on the mainland. Because many amphibians have accelerated evolutionary
rates, some of the already identified species may have unique genetic characteristics, which could lead to the recognition of new species for science. As a conservation measure, it is suggested to allocate the areas with the highest density of species, to areas with restricted access and thus guarantee the survival in the long term for these Critically Endangered species.
Acknowledgment
We thank to our local assistants Vitalio Santiago y Emerson Santiago from Tobobe, Mariano Palacios and Héctor Santiago from Río Caña, who share with us their knowledge about the island and support the field work. Special thanks to Alicia Ibañez, the leader of the project Biodiversity of Escudo, for her passion and encouraging us to continuing working on the island. The Secretaría Nacional de Ciencia y Tecnología (SENACYT) through the projects Escudo de Veraguas Biodiversity and Anfibios en la Unión de las Américas (Contrato por Mérito No. 104-2018-4-FID17-117) funded this work and the bachelor thesis of Olmedo Santiago and Astrid Lizondro, with the themes: the herpetofauna from Escudo de Veraguas Island, and thermoecology of the C. ranoides respectively. The Sistema Nacional de Investigación of the SENACYT founded Abel Batista.
References
Coates, A. G. , McNeill, D. F, Aubry, M.P., Berggren, W. A. , Collins LS. (2005). An introduction to the geology of the Bocas del Toro Archipelago, Panama. Caribbean Journal of Science 41: 374–391.
García-París, M., Wake, D. B. (2000). Molecular phylogenetic analysis of relationships of the tropical salamander genera Oedipina and Nototriton, with descriptions of a new genus and three new species. Copeia 2000: 42–70.
Heyer, W. R., Donnelly, M. A., McDiarmid, R. W., Hayek, L. C., Foster, M. S. (2001). Medición y monitoreo de la diversidad biológica, métodos estandarizados para anfibios. Editorial Universitaria de la Patagonia, Universidad Nacional de la Patagonia San Juan Bosco, Argentina.
Hines, J. E. (2006). PRESENCE- Software to estimate patch occupancy and related parameters. USGS-PWRC. http://www.mbr-pwrc.usgs.gov/software/presence.html.
IUCN SSC Amphibian Specialist Group. 2020a. Oedipina maritima. The IUCN Red List of Threatened Species 2020: e.T59317A54355296. https://dx.doi.org/10.2305/IUCN.UK.2020-3.RLTS.T59317A54355296.en. Downloaded on 16 September 2021.
IUCN SSC Amphibian Specialist Group. 2020b. Craugastor ranoides. The IUCN Red List of Threatened Species 2020: e.T56901A54351272. https://dx.doi.org/10.2305/IUCN.UK.2020-2.RLTS.T56901A54351272.en. Downloaded on 15 September 2021.
MacKenzie, D. I., Nichols, J. D., Lachman, G. B., Droege, S., Royle, J. A., Langtimm, C. A. (2002). Estimating site occupancy rates when de-tection probabilities are less than one. Ecology 83: 2248–2255.
Puschendorf, R., Wallace, M., Chavarría, M. M., Crawford, A. J., Wynne, F., Knight, M., ... Price, S. J. (2019). Cryptic diversity and ranavirus infection of a critically endangered Neotropical frog before and after population collapse. Animal Conservation 22: 515–524.
Zumbado-Ulate, H., Bolanos, F., Willink, B., Soley-Guardia, F. (2011). Population status and natural history notes on the critically endangered stream dwelling frog Craugastor ranoides (Craugastoridae) in a Costa Rican tropical dry forest. Herpetological Conservation and Biology 6: 455–464.