

| Rogemif Fuentes 1 | Erick Barría 2 | Michelle Quiroz-Espinoza 1, 2 |
| https://orcid.org/0000-0002-4389-2665 | https://orcid.org/0000-0001-5677-1590 | https://orcid.org/0000-0002-6955-4605 |
| Eduardo Zambrano 3 | John Cleghorn 4 | Yostin Añino 5 |
| https://orcid.org/0000-0002-4339-8114 | https://orcid.org/0009-0001-4814-982X | https://orcid.org/0000-0002-8870-8155 |
| 11Fundación Los Naturalistas, P.O. Box 0426-01459, David, Chiriquí, Panamá |
| 2Universidad Autónoma de Chiriquí. Instituto Interdisciplinario de Investigación e Innovación |
| 3Universidad de Panamá, Centro regional Universitario de Veraguas, Ciudad de Veraguas, Panamá |
| 4Universidad de Panamá. Centro de investigación e información en medicamentos y tóxicos, Panamá |
| 5Universidad de Panamá. Museo de Invertebrado G.B.Fairchild, Panamá |
| Corresponding autor: rogemifdaniel@gmail.com |
| Enviado el: Enviado el 30 de abril de 2024. Aceptado el 28 de junio de 2024 |
| https://doi.org/10.59722/rcvn.v2i1.739 |
The reproduction of Neotropical snakes, such as those of the genus Leptodeira, varies widely influenced by climatic factors, with species ranging from strictly seasonal to continuous reproductive patterns. Recent research reveals significant variability in reproductive cycles, determined by local environmental conditions such as temperature and precipitation. Nesting behaviors remain poorly understood, and snakes use diverse environments, from natural shelters to communal nests of other species. Within the family Dipsadidae, species such as L. maculata and L. punctata demonstrate correlations between female body size (SVL) and clutch size, reflecting adaptive strategies to maximize reproductive success in diverse ecological contexts. Field work in Panama provided new insights, documenting egg-laying and gravid individuals of species such as L. rhombifera and L. ornata, highlighting further research to elucidate the reproductive dynamics and ecological adaptations particular to each. species. Analytical approaches including Pearson and Spearman correlations and cluster analysis reveal distinct patterns in clutch size relative to female size, underscoring species adaptations and variability within the genus.
Body size, neotropical snakes, oviposition, sexual cycle, snake eggs.
La reproducción de las serpientes neotropicales, como las del género Leptodeira, varía ampliamente influenciada por factores climáticos, con especies que van desde patrones reproductivos estrictamente estacionales hasta continuos. Investigaciones recientes revelan una variabilidad significativa en los ciclos reproductivos, determinada por las condiciones ambientales locales como la temperatura y las precipitaciones. Los comportamientos de anidación siguen siendo poco conocidos, y las serpientes utilizan diversos entornos, desde refugios naturales hasta nidos comunitarios de otras especies. Dentro de la familia Dipsadidae, especies como L. maculata y L. punctata demuestran correlaciones entre el tamaño corporal de la hembra (SVL) y el tamaño de la nidada, lo que refleja estrategias adaptativas para maximizar el éxito reproductivo en diversos contextos ecológicos. El trabajo de campo en Panamá proporcionó nuevos conocimientos, al documentar la puesta de huevos y los individuos grávidos de especies como L. rhombifera y L. ornata, destacando la necesidad de realizar más investigaciones para dilucidar la dinámica reproductiva y las adaptaciones ecológicas particulares de cada especie. Los enfoques analíticos que incluyen correlaciones de Pearson y Spearman y análisis de conglomerados revelan patrones distintos en el tamaño de las nidadas en relación con el tamaño de las hembras, lo que subraya las adaptaciones de cada especie y la variabilidad dentro del género.
Ciclo sexual, huevos de serpiente, oviposición, serpientes neotropicales, tamaño del cuerpo.
Reproduction is a critical event for species and represents an important energetic cost, mainly in ectotherms (Shine, 2003; Vitt & Caldwell, 2013; Bellini et al., 2019). In snakes, the reproductive mode is delimited according to family, and they reproduce by laying eggs (oviparous) or they can give birth to live young (viviparous) (Feldman et al., 2015), with oviparity being the most common mode among reptiles in general (Shine, 1985).
Research on snake reproduction has focused mainly on regions with temperate climates with relatively cold zones, where species usually reproduce during the warmer seasons, because of this, little follow-up has been given to the reproductive biology of species from warmer climates, especially those that live in neotropical regions (Pizzatto et al., 2008a; Scartozzoni et al., 2009). Previous reviews held the belief that most tropical snakes have a continuous, annual reproductive pattern (Fitch, 1970); however, it is known that the reproductive patterns of neotropical snakes vary widely, ranging from strictly seasonal to broadly seasonal and even continuous with periods of increased reproductive activity (Shine, 1991; Pizzatto & Marques, 2002). However, recent research has shown that in some species, the reproductive cycle of females is seasonal while it is continuous in males, suggesting a reproductive strategy adapted to the tropical environment where water levels and prey availability play a crucial role (García-Cobos et al., 2020). Temperature, precipitation, and humidity are the main abiotic factors that influence these cycles; therefore, they are responsible for modulating their seasonality (Aldridge & Duvall, 2002; Lutterschmidt & Mason, 2009).
Most snakes cannot build a nest and depend on pre-existing sites for their oviposition: under rocks, logs or any other surface cover, in preformed underground chambers, nests of other animals such as alligators, ants and termites, however, in solitary or communal nests, the nesting sites and oviposition modes of Neotropical snakes are poorly understood, due to the success of mothers in hiding their eggs (Baer et al., 2009; Blouin-Demers et al., 2004; Braz et al., 2008; Sierra-Serrano et al., 2023).
The Family Dipsadidae has the largest radiation of colubrid snakes in the Neotropics, with approximately 700 species present mainly in Central and South America (Stender-Oliveira et al., 2016). Within it is the genus Leptodeira belonging to the tribe Leptodeirini, which is composed of relatively large aglyph or opistoglyph and oviparous snakes (Pizzatto et al., 2008a). There are records of clutches for five species of Leptodeira in Central America and Mexico (Kohler et al. 2016; Nieto-Toscano & Martínez-Coronel, 2021; Sierra-Serrano et al., 2023).
We currently consider the taxonomy suggested by Costa et al. (2022) to be valid. who propose for Panama the presence of three species of cat's eye snakes, one of them requires description, Leptodeira septentrionalis ornata (samples from Bocas del Toro and Costa Rica) raised as a putative “Not described” species that must remain as a subspecies until sampling is expanded; they elevate L. annulata rhombifera to a species as suggested by Savage (2002) and Barrios-Amorós (2019), they redefine and recognize L. ornata as a species in populations of Colombia and southern Panama.
For this work we carried out an analysis of important points such as the relationship between body size and clutch size, this relationship can be important how reproductive estrategy and we sought to expand information on the clutches of cat's eye snakes of the genus Leptodeira in Panama. In the tropics, considering that despite being relatively common (Solórzano, 2004), they have few records of laying or clutches of eggs, most of them are associated with accidental clutches of snakes captured for other types of studies or occasional encounters on field.
An exhaustive bibliographic review was carried out to compile all reports of reproduction in snakes of the genus Leptodeira throughout its distribution, including the island territory. We had access to articles published in the following journals: Amphibia-Reptilia, Herpetological Conservation and Biology, Herpetological Monographs, Herpetological Review, Herpetology Notes, Herpetological Bulletin, Mesoamerican Herpetology, Reptiles & Amphibians, and Herpetologica, using “Google scholar” (https://scholar.google.com/schhp?hl=en) and “ ResearchGate” (https://www.researchgate.net/) to search for articles published until October 2023, using the combination of the following keywords: “reproduction+americans+Colubridae”, “reproduction+americans+Dipsadinae”, “Leptodeira´s+reproduction” and “American+cat-eyed snakes'+reproduction”.
During some field work with different objectives, four female cat's-eye snakes (Leptodeira spp.) were collected and placed in captivity, which resulted in the laying of eggs by three of them and the entry of a gravid female to the collection of the Herpetological Museum of the Universidad Autónoma de Chiriquí.
Report 1. On October 10th, 2022, Eduardo Leiva rescued a female L. rhombifera in Panama City, Panama province, which was kept in captivity by JC. This individual laid three eggs on October 11th, 2022, and they were incubated for 60 days in a medium that consisted of a container with dry vermiculite and closed with some holes in the lid, this was placed inside another closed container that contained approximately 1 cm of water at temperature, between 27 and 30 degrees during the day and 22 to 24 degrees at night, with relative humidity between 80 and 90 %, the hatching success was 100 %, one of them escaped and the others were released in the Soberanía National Park (figure 1A).
Report 2. During a field trip of the project “Revisión filogenética y taxonómica de las serpientes ojo de gato (Leptodeira spp.) en Panamá” led by the main author of this work, on June 7th, 2023, two female specimens of L. ornata in the Salto del Mono camp, Portobelo National Park, Colón province, were kept in captivity for a week for processing as part of the study and on June 14th, 2023. One snake (collection code RF-011, museum code MHCH-5012) laid a clutch of seven eggs, which were placed in an incubation system like that in report 1. After a week, the eggs underwent ovoscopy, revealing no signs of development. Despite being kept in the incubation system, they failed to develop (figure 1B).
Report 3. On August 13th, 2023, a female L. rhombifera was rescued in Santiago city, Veraguas province, which was kept in captivity to be photographed and relocated. On August 28th, this individual laid one egg and another six on September 4, 2023, all eggs were placed in an incubation system like that in report 1, however, after an ovoscopy it was determined that they were infertile (figure 1C).
Report 4. On December 6th, 2023, a female L. rhombifera was rescued in Divisa city, Veraguas province, by Abel Batista, and introduced in the Herpetological Museum of the Universidad Autónoma de Chiriquí, where the biologist MQ was able to observe that the female (collection code RF-015, museum code MHCH-5016) was pregnant. To verify this, a necropsy was performed that allowed observation. We placed the animal in a supine position and made a paraventral linear cut in the second third of the snake's body with a sterile scalpel (Pessier & Pinkerton, 2003). Four eggs could be observed (figure 1D).
The Shapiro-Wilk test was employed to assess the normality of all SVL, and egg number values obtained from the bibliographic review and new records and the homoscedasticity was assessed using the Breusch-Pagan test. Subsequently, Pearson correlation analyses were conducted using RStudio Software (version 4.2.2) to examine the relationship between snout-vent length (SVL) and the number of eggs per clutch. Additionally, separate Pearson correlation analyses were performed for SVL, and the number of eggs laid by L. maculata and L. punctata species.
In cases where literature only provided total length rather than SVL, we estimated SVL using tail length data from other sources. We also attempted a Pearson correlation analysis between average egg size and clutch size based on available literature data.
Finally, to explore the variation in clutch size and SVL between different species of the genus Leptodeira, we performed a similarity cluster using Euclidean distances and Paired group algorithm (UPGMA) that analyzes all species individually
Bibliographic review We managed to locate 43 records, in total 10 of L. maculata with clutches between 4 - 11 eggs, five of L. frenata between 2 - 7 eggs, 12 of L. punctata between 6 -11 eggs, two of L. uribei with five and six eggs, four of L. ashmeadii between 4 - 6 eggs, four L. s. pilysticta between 6 – 12 eggs, four L. ornata with four eggs and we are providing two new reports of clutches of L. rhombifera in captivity and one gravid female for this specie, and one for L. ornata (table 1).
Table 1. Clutch size records by species of genus Leptodeira.| Species of Genus Leptodeira | Clutch Size | Country | SVL (mm) | Egg Length - Width (mm) | Record | Reference |
| L. maculata | 5 | Mexico | 510.72 | 28.0 ─ 13.0 | Captivity | Duellman, 1958 |
| L. maculata | 7 | Mexico | - | - | Captivity | Duellman, 1958 |
| L. ornata | 4 | - | 559.12 | 34.5 ─ 11.7 | - | Duellman, 1958 |
| L. septentrionalis polysticta | 7 | - | 726.1 | 28.9 ─ 12.0 | - | Haines, 1940 |
| L. s. polysticta | 6 | - | 726.1 | 25.7 ─ 10.8 | - | Haines, 1940 |
| L. s. polysticta | 9 | - | 726.1 | 23.7 ─ 11.8 | - | Haines, 1940 |
| L. s. polysticta | 12 | - | 726.1 | 21.1 ─ 11.8 | - | Haines, 1940 |
| L. maculata | 9 | Mexico | - | - | Captivity | Ramírez-Bautista, 1994 |
| L. maculata | 7 | Mexico | 533 | - | Captivity | Goldberg, S. R., 2004 |
| L. maculata | 11 | Mexico | 630 | - | Captivity | Goldberg, S. R., 2004 |
| L. maculata | 8 | Mexico | 503 | - | Captivity | Goldberg, S. R., 2004 |
| L. maculata | 8 | Mexico | 519 | - | Captivity | Goldberg, S. R., 2004 |
| L. maculata | 4 | Mexico | 480 | - | Captivity | Goldberg, S. R., 2004 |
| L. maculata | 8 | Mexico | 527 | - | Captivity | Goldberg, S. R., 2004 |
| L. maculata | 10 | Mexico | 573 | - | Captivity | Goldberg, S. R., 2004 |
| L. frenata | 7 | Mexico | 604 | - | Captivity/dissected | Kohler et al., 2016 |
| L. frenata | Mexico | 2 | - | - | Captivity | Kohler et al., 2016 |
| L. frenata | Mexico | 4 | - | - | Captivity | Kohler et al., 2016 |
| L. frenata | Mexico | 7 | - | - | Captivity | Kohler et al., 2016 |
| L. frenata | Guatemala | 7 | - | - | Captivity/dissected | Stuart, 1935 |
| L. punctata | 6 | Mexico | 435.05 | - | Captivity | Duellman, 1958 |
| L. punctata | 6 | Mexico | - | - | Captivity | Hardy & McDiarmid, 1969 |
| L. punctata | 7 | Mexico | - | - | Captivity | Hardy & McDiarmid, 1969 |
| L. punctata | 9 | Mexico | 468 | - | Captivity | Goldberg, 2004 |
| L. punctata | 8 | Mexico | 481 | - | Captivity | Goldberg, 2004 |
| L. punctata | 8 | Mexico | 422 | - | Captivity | Goldberg, 2004 |
| L. punctata | 6 | Mexico | 371 | - | Captivity | Goldberg, 2004 |
| L. punctata | 9 | Mexico | 428 | - | Captivity | Goldberg, 2004 |
| L. punctata | 6 | Mexico | 382 | - | Captivity | Goldberg, 2004 |
| L. punctata | 7 | Mexico | 393 | - | Captivity | Goldberg, 2004 |
| L. punctata | 11 | Mexico | 523 | - | Captivity | Goldberg, 2004 |
| L. punctata | 6 | Mexico | 410 | - | Captivity | Goldberg, 2004 |
| L. uribei | 5 | Mexico | 488 | - | Dead snake/Dissected | Martínez-Coronel, 2021 |
| L. uribei | 6 | - | 480 | - | - | Streicher et al., 2011 |
| L. ashmeadii | 6 | Colombia | - | - | Ant nest Acromyrmex santschii | Sierra-Serrano et al., 2023 |
| L. ashmeadii | 4 | Colombia | - | - | Hollow trunk | Sierra-Serrano et al., 2023 |
| L. ashmeadii | 6 | Colombia | 583 | 27.08 ─ 14.50 | Captivity | Sierra-Serrano et al., 2023 |
| L. ashmeadii | 6 | Colombia | 583 | 26.8 ─ 16.6 | Captivity | Sierra-Serrano et al., 2023 |
| L. rhombifera | 5 | Panama | - | - | Ant nest Atta colombicaBaer et al., 2009 | |
| L. rhombifera | 3 | Panama | - | - | Captivity | This study |
| L. ornata | 7 | Panama | 488.96 | 31 ─ 12.3 | Captivity | This study |
| L. rhombifera | 7 | Panama | 480 | 22.1 ─ 11.64 | Captivity | This study |
| L. rhombifera | 4 | Panama | 411.78 | 29.34─10.58 | Captivity/dissected | This study |
The correlation analysis between SVL and number of eggs per clutch resulted in a r=0.29, and a p>0.05, which indicates that, at the genus level, there is statistically no correlation (figure 2 Above). When carrying out the same analysis with the two species that had the most information available in the literature, L. maculata (10) and L. punctata (12), the result was r=0.86 (figure 2 Left) and p<0.05 and r=0.82 (figure 2 Right), p<0.05, respectively, demonstrating in these two species a strong correlation between the SVL of mothers with the number of eggs per clutch (figure 2 Bottom). When analyzing the correlation between the average size and the number of eggs per clutch, we found an inverse and moderate correlation, with r=-0.56, p<0.05.

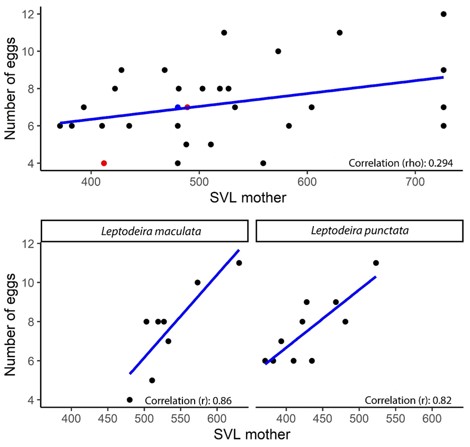
Figure 2. Above: Spearman correlation graph shows the correlation between the number of eggs laid per clutch and the snout-vent length (SVL) of the mothers (blue dot for L. ornata and red dots for L. rhombifera). Bottom: Pearson correlation graphs show the correlation between the number of eggs laid per clutch and the SVL of the mothers, applied to L. maculata (Left) and L. punctata (Right).
Through the cluster we can notice a marked separation in terms of the relationship between clutch size and egg size per species, with L. septentrionalis polysticta representing an isolated group and the one with the highest correlation, the rest of the species form other groups with relatively homogeneous (figure 3).
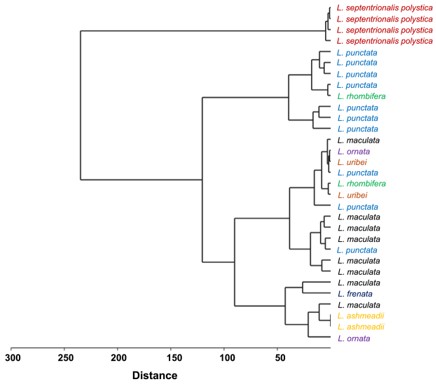
Figure 3. Similarity cluster using Euclidean distances and Paired group algorithm (UPGMA) for the relationship corresponding to the number of eggs and the snout-vent length (SVL) of the mothers for the different species of genus Leptodeira.
The number of eggs showed normality, while the SVL of the mothers did not, leading to the use of Spearman's rho correlation instead of Pearson correlation to analyze the relationship between these two factors. The separate analyses for each species (Leptodeira maculata and Leptodeira punctata) exhibited normality in the data, hence Pearson correlation was applied in these cases.
Spearman's rho correlation analysis performed with all species present in this work showed no significant correlation between SVL and egg number (figure 2 above). However, some species differ in body length to reach their reproductively active state (García-Cobos et al., 2020). Clutch size usually has a positive correlation with body size; As we observed in our results for the species L. maculata and L. punctata with correlations between the number of eggs and the length of the SVL of females, however, several factors can affect clutch size (Shine, 1994), this This statement is represented by the Pearson correlation between these variables, which, although low, shows that the larger the clutch, the smaller the size of the eggs (King, 1989).
The hierarchical analysis of the cluster allows us to observe which species have the greatest relationship concerning female size (SVL) and clutch size, suggesting possible common evolutionary adaptations or similar ecological niches. L. septentrionalis polystica clusters apart (figure 3), indicating that there is no correlation between the size of females of this species and clutch size, this is supported by the records of Haines (1940) where with the same size different clutch sizes occur, unlike the other species studied that form other relatively homogeneous groups such as L. punctata, while species such as L. maculata and L. rhombifera appear more dispersed, which reflects greater variability. Furthermore, L. ashmeadii, L. uribei, L. rhombifera and L. ornata form another group, which suggests similarities in the variables analyzed, that is, as the SVL increases, the number of eggs or clutch size also increases (figure 3), the condition presented by L. septentrionalis polystica allows us to think that throughout the growth of the female it is possible to reach a limit point where there is no longer a correlation between SVL and clutch size.
Because this analysis was performed on the few individuals of the different species that had egg size data, this analysis may be biased. In most snakes, the female is usually larger than the male, this is known as sexual dimorphism and the males usually have a longer tail due to the presence of hemipenes and their retractor muscles (King, 1989). However, in many arboreal snakes this dimorphism is absent (Fowler & Salomão, 1994; Pizzatto & Marques, 2007). Nevertheless, body size has a high cost, since the mobility of laying females is reduced and they are more susceptible to predation: the greater the number of eggs, the greater the cost (Shine, 1980). Each sex adopts a different strategy: males are subject to sexual selection and tend to spend time and energy searching for potential mates, fighting other males, while females tend to increase their fecundity by dedicating time and energy to reproductive strategies that help them allow increasing the size of clutches and the frequency of reproduction (Madsen & Shine 1994; Barbosa et al. 2022).
According to Mathies (2011), snakes must be analyzed at the individual level, classifying them as: discontinuous cyclical, continuous or acyclical and at the population level, as seasonally synchronous, semi-synchronous or unseasonal. In tropical regions, snake populations tend to have continuous rather than seasonal reproductive cycles due to the relatively stable climate (Duellman, 1958; Fitch, 1982; Vitt & Vangilder, 1983). In contrast to this, most Dipsadidae reproduce seasonally, females show gravidity/pregnancy and oviposition/parturition mainly in the rainy season, but the duration is variable between species (Pizzatto & Marques, 2002; Pizzatto et al. 2008b; Mathies, 2011), this is consistent with our records, since they all cover from June to December, which coincide with the rainy season of Panama, so we can think of seasonal cycles for the species L. rhombifera and L. ornata, although we consider the possibility that this assertion will change with more records. Despite this, Maschio et al. (2021) emphasizes the importance of analyzing different populations of the same species separately, given the influence of environmental and geographical factors on their reproductive and survival strategies
The findings of Pizzatto et al. (2008a) on the extended follicular cycles in L. annulata indicate a capacity for year-round reproduction, even in seasonal climates, contrasting with the seasonal and discontinuous pattern observed in females of other species, as mentioned by Callard & Kleis (1987) and Mathies (2011). These differences suggest divergent adaptations to environmental and energetic pressures between sexes and species. Moreover, a study on the genus Elaphe revealed that although residual reproductive rate increases with body length in E. longissima, this pattern was not observed in E. quatuorlineata, implying distinct responses to environmental conditions and resource stability. Additionally, differences in the average clutch size between these species indicate that E. longissima might be adapted to produce larger clutches, possibly as a strategy related to its body size rather than the age of the females, contrasting with the stability observed in E. quatuorlineata and other Mediterranean populations (Capizzi et al., 1996). These findings underscore the complexity of reproductive strategies and the importance of considering both environmental factors and intrinsic species characteristics to understand variability in reproductive cycles and population dynamics in snakes.
There is no statistically significant correlation between SVL and clutch size when analyzed together, however, when analyzing the samples independently there is such a correlation, being more marked in L. s. polystica than in other species.
Both in the cluster and in the PCA L. s. polystica as a distinct group with a directly proportional relationship between the variables, L. maculata and L. punctata show a tendency towards homogenization, for this reason, we consider that by increasing the number of records the groups will be more isolated, allowing correlations to be established by species.
The premise that the greater the SVL, the greater the number of eggs or clutch size is fulfilled for all cases except for L. septentrionalis polystica according to the hierarchical analysis.
We thanks to SENACYT for financing the project “Revisión filogenética y taxonómica de las serpientes ojo de gato (Leptodeira spp.) en Panamá” Where did we get the individual RF-011, to Ministerio de Ambiente de Panamá for the corresponding collection permit, to the Instituto Interdisciplinario de Investigación e Innovación (I4) for providing the laboratory for the necropsy of specimen RF-015, to Jesse Aschcroft for their work in the field and collection of specimens RF-011 and Abel Batista for the collection of specimen RF-015.